Radium
88
Ra
Gruppe
2
Periode
7
Block
s
Protonen
Elektronen
Neutronen
88
88
138
Generelle Eigenschaften
Ordnungszahl
88
Atommasse
[226]
Massenzahl
226
Kategorie
Erdalkalimetalle
Farbe
Silber
Radioaktiv
Ja
From the Latin word radius meaning ray
Kristallstruktur
Kubisch raumzentriert
Geschichte
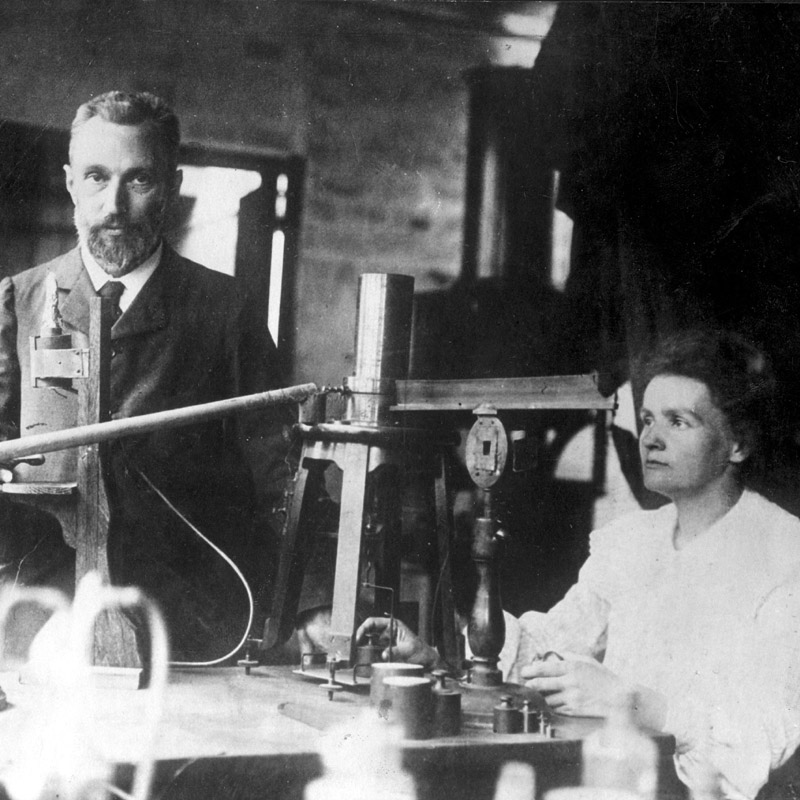
Radium was discovered by Marie Curie and Pierre Curie in 1898.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
Elektronen pro Schale
2, 8, 18, 32, 18, 8, 2
Elektronenkonfiguration
[Rn] 7s2
Radium imparts a carmine red color to a flame
Physikalische Eigenschaften
Aggregatzustand
Fest
Dichte
5,5 g/cm3
Schmelzpunkt
973,15 K | 700 °C | 1292 °F
Siedepunkt
2010,15 K | 1737 °C | 3158,6 °F
Schmelzwärme
8 kJ/mol
Verdampfungswärme
125 kJ/mol
Spezifische Wärmekapazität
-
Häufigkeit in der Erdkruste
9,9×10-12%
Häufigkeit im Universum
n/a
CAS-Nummer
7440-14-4
PubChem CID-Nummer
6328144
Atomeigenschaften
Atomradius
-
Kovalenter Radius
221 pm
Elektronegativität
0,9 (Pauling-Skala)
Ionisierungsenergie
5,2784 eV
Molares Volumen
45,20 cm3/mol
Wärmeleitfähigkeit
0,186 W/cm·K
Oxidationszustände
2
Anwendung
Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium is highly radioactive and carcinogenic
Isotope
Stabile Isotope
-Instabile Isotope
202Ra, 203Ra, 204Ra, 205Ra, 206Ra, 207Ra, 208Ra, 209Ra, 210Ra, 211Ra, 212Ra, 213Ra, 214Ra, 215Ra, 216Ra, 217Ra, 218Ra, 219Ra, 220Ra, 221Ra, 222Ra, 223Ra, 224Ra, 225Ra, 226Ra, 227Ra, 228Ra, 229Ra, 230Ra, 231Ra, 232Ra, 233Ra, 234Ra